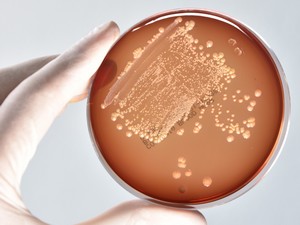
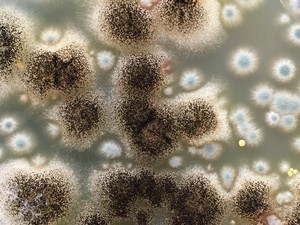
Antibacterial and Antimicrobial Tests
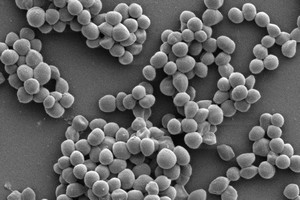
The selection of the ideal test method for antimicrobial properties depends on many factors, e.g. material properties, absorbency, water content, hydrophilicity, hydrophobicity, surface structure, prototype/product size, etc.
In addition, the choice of suitable test organisms, which can be found in the application areas in addition to the standard test organisms, is important. Our laboratory therefore has a large collection of strains of test organisms, including many resistant and multi-resistant variants, e.g. MRSA, MRSE, VRE, ESBL, MRGN strains.
We would be happy to advise you on the selection of the most suitable test methods for your product in your application.
ISO 22196: Measurement of antibacterial activity on plastics and other non-porous surfaces
JIS Z 2801: Antibacterial products – Test for antibacterial activity and efficacy
ASTM E 2180: Standard Test Method for Determining the Activity of Incorporated Antimicrobial Agent(s) In Polymeric or Hydrophobic Materials
ASTM E 2149: Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents Under Dynamic Contact Conditions
ASTM E 2315: Assessment of Antimicrobial Activity Using a Time-Kill Procedure
ISO 20743: Textiles – Determination of antibacterial activity of textile products
AATCC 100: Antibacterial Finishes on Textile Materials: Assessment of
ISO 7581: Evaluation of bactericidal activity of a non-porous antimicrobial surface used in a dry environment
DIN 58940-3: Susceptibility testing of microbial pathogens to antimicrobial agents – Part 3: Agar diffusion test
DIN EN ISO 11737: Part 2: Tests of sterility performed in the definition, validation and maintenance of a sterilization process
DIN EN 16615: Chemical disinfectants and antiseptics – Quantitative test method for the evaluation of bactericidal and yeasticidal activity on non-porous surfaces with mechanical action employing wipes in the medical area (4-field test) – (phase 2, step 2)
Proliferation Assay: Proliferation-based assay for the determination of antimicrobial surface properties
Mixed-Culture Assay: Differential analysis of bacterial adhesion, proliferation and antimicrobial efficacy of material surfaces against mixed cultures of bacteria
DIN EN 1276: Chemical disinfectants and antiseptics – Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic and institutional areas
DIN EN 1650: Chemical disinfectants and antiseptics – Quantitative suspension test for the evaluation of fungicidal or yeasticidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic and institutional areas
DIN EN 13727: Chemical disinfectants and antiseptics – Quantitative suspension test for the evaluation of bactericidal activity in the medical area – Test method and requirements (phase 2, step 1)
Antimycotic Fungal Test Methods
The fungus tests examine the resistance of materials to different fungi or mixtures of fungi. Material properties such as chemical composition, absorbency, water content, hydrophilicity, hydrophobicity, surface structure or prototype/product size also play an important role here.
We would be happy to advise you on the selection of the most suitable test methods for your product in your application.
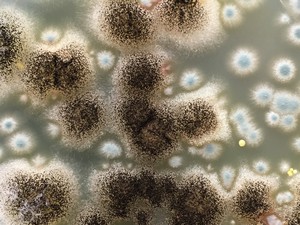
DIN EN ISO 846: Plastics – Evaluation of the action of microorganisms (Method A and B)
ASTM G 21: Standard Practice for Determining Resistance of Synthetic Polymeric Materials to Fungi
BS 3900 G6: Assessment of resistance to fungal growth
ISO 13629-2: Textiles – Determination of antifungal activity of textile products – Part 2: Plate count method
DIN EN 15457: Paints and varnishes – Laboratory method for testing the efficacy of film preservatives in a coating against fungi
Leak testing of medical devices
The tightness of medical products ensures that no chemical components can escape from the systems and that no microbial contamination can enter the systems during use. This is a critical aspect, especially for medical devices that have direct access to patients and involve manual intervention during use.
In addition to standard methods, the laboratory has developed test procedures specifically for these requirements.
We would be happy to advise you on the selection of the most suitable test methods for your product in your application.

DIN EN ISO 24072: Aerosol bacterial retention test method for air-inlet on administration devices
YY/T 1551.1: Air filters for medical infusion and transfusion equipment – Part 1: Aerosol bacterial retention test method
Aerosol Contamination Test: Microbial Barrier Testing by means of Bacterial Aerosol Contamination
Touch Contamination Test: Microbial Barrier Testing by means of Bacterial Touch Contamination
Chemical Tightness Test: Closed System Test by means of a fluorescent tracer
DIN 58953-6: Sterilization – Sterile supply – Part 6: Microbial barrier testing of packaging materials for medical devices which are to be sterilized